
Pharmacokinetics
Pharmacokinetics
Inovative Technology Support Group for Life Science and Drug discovery
Test Introduction
Pharmacokinetic (PK) analysis refers to the field of study that uses mathematical calculations to work on the quantitative understanding of the overall absorption, distribution, metabolism, and excretion processes over time since the medicine is injected inside the human body. The tests evaluate the movements of the test material from its exposure in human body to excretion at a certain concentration or dose in which the medicinal efficacy is expected. By measuring the drug concentration in blood, tissues, urine and feces to calculate the PK parameter and information necessary to set the clinical dose is given based on the obtained data. The LC-MS based PK study of the Proteinworks provides the most accurate information regarding the antibody protein drugs.
Sample
- Antibody
- Antibody drug conjugates
- Peptides
- Recombinant proteins
Analysis Process
- Experimental
animals - Drug
administration - Sampling
- Analysis of drug
concentration(LC-MS/MS) - PK parameter
calculation
Analysis Result
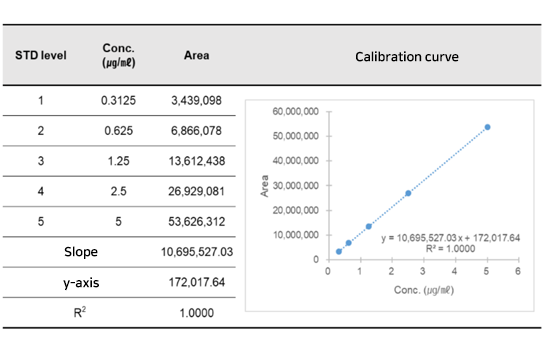
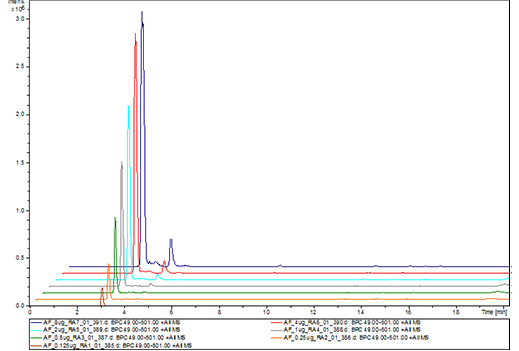
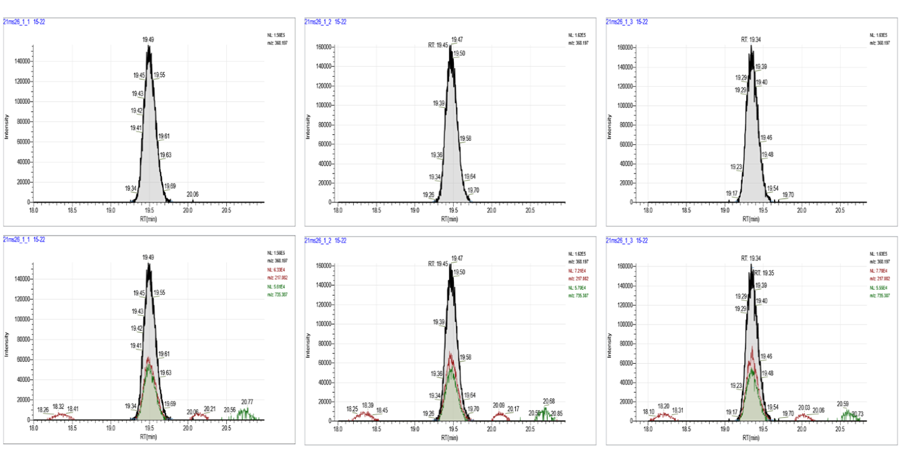
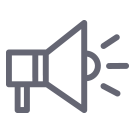
Pharmacokinetics study
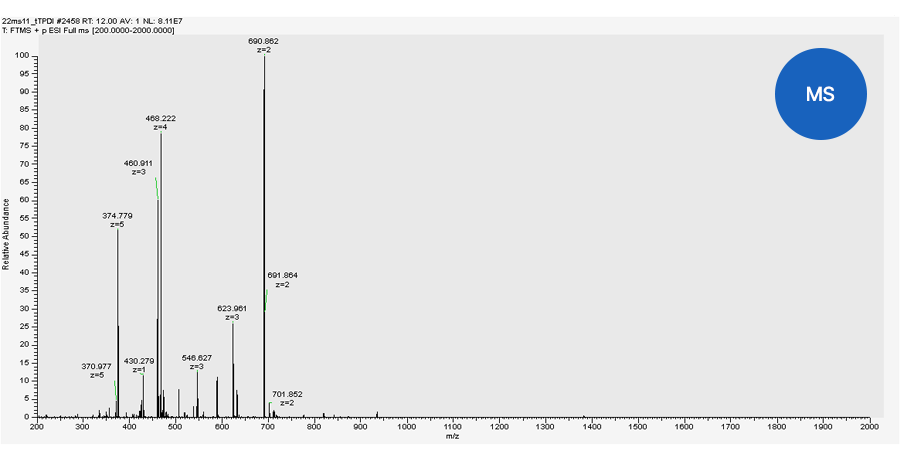
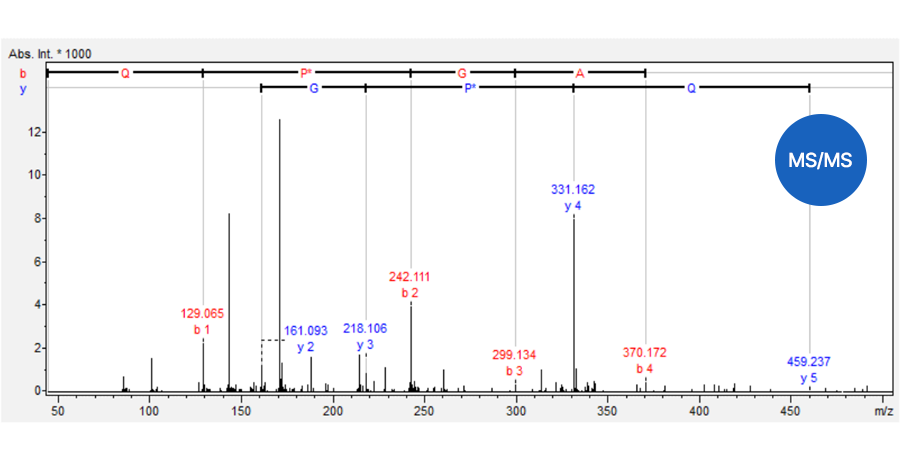
Quantitative analysis by TSQ-MS
- Download Your Request
 Analysis Consulting : 042-384-0702, 070-5014-5710
Analysis Consulting : 042-384-0702, 070-5014-5710